
 |
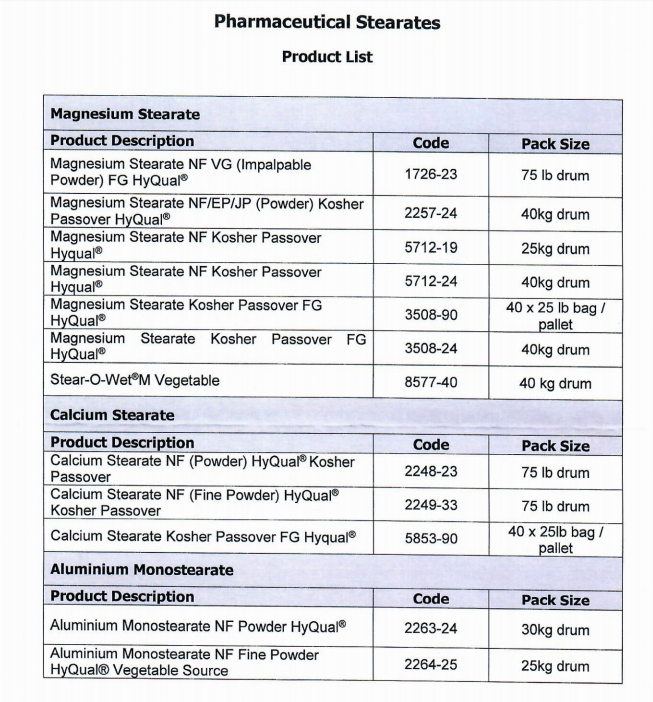
Mallinckrodt Magnesium Stearate |
Mallinckrodt (SpecGx)'s Magnesium Stearate
Magnesium Stearate Code 5712
CFDA Filing Code: F20170000131 "A" status
Criterions: MEETS NATIONAL FORMULARY (NF) SPECIFICATIONS
MEETS EUROPEAN/BRITISH PHARMACOPEIA (EP/BP) SPECIFICATIONS
MEETS CHINESE PHARMACOPEIA (ChP) SPECIFICATIONS
MEETS JAPANESE PHARMACOPEIA (JP) SPECIFICATIONS
Applications:It is used as excipient of pharmaceutical applications and food additives.
Magnesium Stearate Code 2257
CFDA Filing Code: F20180001423 "A" status
Criterions: MEETS NATIONAL FORMULARY (NF) SPECIFICATIONS
MEETS EUROPEAN/BRITISH PHARMACOPEIA (EP/BP) SPECIFICATIONS
MEETS CHINESE PHARMACOPEIA (ChP) SPECIFICATIONS
MEETS JAPANESE PHARMACOPEIA (JP) SPECIFICATIONS
Applications:It is used as excipient of pharmaceutical applications and food additives.
Since 2007, our company has been the agent and distributor in China for Mallinckrodt (SpecGx), a prominent global excipient manufacturer in the field of pharmaceutical products whose magnesium stearate has been used in several dozen types of tablets worldwide. Magnesium stearate codes 2257 and 5712 are published on CFDA's registration platform under DMF No. F20180001423 & F2017000131 with approval statuses.
 Hotline
0086-21-65128389
Mobile: 13901999810
Hotline
0086-21-65128389
Mobile: 13901999810